Comparative Study on Macrobenthic Community Structure with Special Reference to oligochaetes during Drought and Flooded Phases in a Tropical Kole Wetland, India. 
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2015, Vol. 5, No. 41 doi: 10.5376/ijms.2015.05.0041
Received: 22 Apr., 2015 Accepted: 26 May, 2015 Published: 23 Jun., 2015
Vineetha S., Bijoy Nandan S., and Rakhi Gopalan K.P., 2015, Comparative Study on Macrobenthic Community Structure with Special Reference to Oligochaetes During Drought and Flooded Phases in a Tropical Kole Wetland, India, International Journal of Marine Science, 5(41): 1-10 (doi: 10.5376/ijms/2015.05.0041)
Wetlands experience dynamic hydrofluxes which is a major determinant of biotic community structure. Benthic organisms form an integral part of aquatic environment and constitute an important link in the food web. The habitat loss and fragmentation caused by hydrological alterations in wetlands can affect the benthic fauna. This study analyzed the benthic community especially oligochaete species in a wetland during two different phases. Maranchery Kole wetland, a part of Vembanad Kole wetlands (Ramsar site) behaved as two systems within a short span of one year; as flooded phase when the system was a pure aquatic body and drought phase where the system was dry resembling a grass land with isolated water patches. Macrobenthic abundance in flooded and drought phases were (355±122 ind./m2) and (166±60 ind./m2) respectively. The decrease in habitable area along with its consequential effects resulted in a reduced numerical abundance of macrobenthos in the drought phase. Oligochaete abundance was significantly higher in flooded phase (ANOVA F1, 53=5.11, p<0.05). On the contrary insect abundance was higher in drought phase (124±168 ind./m2) than flooded phase (91±156 ind./m2). As insects are characterized by flight dispersal mode they were less affected by habitat isolation caused by the fragmented water patches. Eighteen oligochaete species were identified consisting of 14 species of the family Naididae, 3 of Tubificidae and 1 of Lumbriculidae. Allonais gwaliiorensis, Dero dorsalis, Branchiodrilus hortensis, Pristinella acuminata, Nais andhrensis, Dero nivea were exclusive to drought phase and Pristina breviseta, Pristinella minuta, Pristinella menoni, Aulophorus hymnae, Lumbriculus variegates exclusive to flooded phase. The ANOSIM results revealed that the oligochaete species composition was similar in both the phases (Global R=0.05, p>0.05). Species richness (Margalef’s index) and species diversity (Shannon Wiener index) was higher in drought phase or disturbed phase than flooded phase or undisturbed phase reflecting a glimpse of intermediate disturbance theory. No significant correlation emerged between the environmental variables and benthic abundance implying that the relationship with the measured environmental variables might be weaker or overridden by other unmeasured variables. Further the generalized environmental requirements in aquatic oligochaetes and chironomid larvae, the most abundant taxa in the wetland could be the reason for the lack of correlation.
Introduction
Wetlands are characterized by hydrological alterations resulting in hydrological disturbances. Due to extreme hydrological fluctuations in wetlands, the aquatic phase is shrunk spatially and temporally. The reduced water level leads to habitat loss and habitat fragmentation especially for aquatic organisms. The loss of habitable area is the predominant cause of population (Hughes et al., 1997) and species extinctions (Pimm et al., 1995). Isolation of fragments and edge effects associated with such fragmentation can cause further declines in both the number of species, changes in their relative abundance and other aspects of biodiversity within the habitat patches (Ewers and Didham, 2006). However an increase in abundance due to reduced water level was also recorded by Martins et al., (2008) and Suriani-Affonso (2011).
The role of benthos as a link between primary producers, decomposers and higher trophic levels in the ecosystem is well known. They form an integral part of aquatic environment and have the capability to integrate the environmental effects due to their sedentary habits and relatively long life span (Pandit et al., 1991). Hydrological alterations in wetlands can affect the benthic fauna directly and indirectly by making the habitat completely inhabitable or reducing the habitable area to a few water patches. Habitat isolation and fragmentation would prevent circulation and interchange of larvae and other propagules between parts of the system; processes governing larval settlement ecology are key structuring agents of benthic communities (Eckman, 1996).
The benthic response to drawdown conditions is also of particular concern as hydrological extremes with enhanced drought and flooding episodes is predicted for many parts of the world in the climate changing scenario (EEA, 2007; IPCC, 2010). The increasing stress placed on wetlands also demand similar studies. India has lost more than 38% of its wetlands in just the last decade, at rates as high as 88% in some districts (Vijayan et al., 2004). Further many of the water bodies in India are temporary, showing large water level fluctuations, exposing the basin to drying (Gopal and Zutshi, 1998). Most of the studies on benthos focus on the seasonal comparison (pre monsoon, monsoon and post monsoon) (Sharma and Rawat 2007; Kumar and Khan, 2013) but those focusing wet and dry phases are scarce. Though in many parts of the world, the comparison of benthos from dry and wet phases were done from field and experimental studies (Sommer and Horwitz, 2009; Nkwoji et al., 2010; Hamilton et al., 2013), the information from India remains scanty.
The present study is from Maranchery kole wetland, a part of Vembanad-Kol wetlands (Ramsar Site), the largest brackish, humid and tropical wetland system in the south west coastal state of Kerala. The area experienced extreme hydrological variability behaving as a dry or grass land with isolated water patches and a pure aquatic body within a span of one year due to agricultural related activities and precipitation. This study tried to explore the difference in macrobenthic composition, abundance, oligochaete species and community structure among the drought (dry or grass land with isolated water patches) and flooded (aquatic body) phases.
1 Materials and Methods
1.1 Study area and the Hydrological regime
The Kole lands are saucer shaped tracts, lying 0.5 to 1.5 m below the mean sea level, covering an area of 13,632 ha. spread over Thrissur and Malappuram districts of Kerala extending from Northern bank of Chalakkudy river in the South to the Southern bank of Bharathappuzha river in the North. The Viyyam dam is situated at the downstream of end of Kole lands which prevents the intrusion of salt water to the Kole lands. The Kole lands are supposed to be lagoons formed by the recession of seas centuries back. A shallow portion of the sea along the western periphery of the main land was isolated and they were gradually silted up during rains making the lagoons shallow (Kurup and Varadachar, 1975). The farmers then bunded the fields, dewatered and raised rice in summer months. During the rains, the inflow into the basin submerges all the kole areas. The area is normally flooded from June to January. The main crop is Punja (summer crop) raised during January to April/May. Towards the close of the North East monsoon, water from the rice fields are pumped out by an indigenous axial flow centrifugal pumping device (petti and para) and sowing or transplanting is done by January. The cyclical nutrient recharging of the wetland during the flood season made the area as one of the most fertile soils of Kerala. Even the word “Kole” is a term in Malayalam (the regional language in Kerala, India) which means ‘bumper yield of high returns in case flood does not damage the crops’ (Johnkutty and Venugopal, 1993).
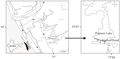
The study area, with an area of 100 acres, is a part of the Ponnani Kole lies in between Maranchery and Veliyamkodu panchayats (a village council is called panchayat) in Malappuram district. A total of five stations were selected for monthly sampling (Figure 1). In January 2010, water was drained from the stations as the preparation for paddy cultivation. In February 2010,these sites were again filled with water due to the breaching of an adjacent earthen bund. Paddy cultivation was not done due the breech of the bund so the land was covered with grass with isolated water patches. The period from January 2010 to June 2010 could be considered as drought phase as it fits into the definition of drought. Drought has been defined as ‘an unpredictable low-flow period, which is unusual in its duration, extent, severity or intensity’ (Humphries and Baldwin, 2003). During this period, the benthic samples were collected from the available small water patches. February 2010 was excluded because it was inundated due to the breach of a bund hence it could not be considered as drought period. These stations were inundated by the end of June 2010 with the advent of South West monsoon. The period from July 2010 to December 2010, the study area behaved as an aquatic ecosystem with an average depth of 2.16 m. So the period from July 2010 to December 2010 was considered as flooded phase (Figure 2).
1.2 Sample collection and analysis
The sediment samples for the analysis of environmental parameters and macrobenthos were collected using a Van Veen grab of size 45cm2. Temperature of the sediment samples were determined in the field using a standard degree centigrade thermometer of the range 0℃ to 50℃ (0.l℃ accuracy). Sediment pH was measured using Systronics water analyzer model 321 (accuracy±0.01) (APHA, 2005). Moisture Content was determined by gravimetric analysis after drying at a maximum temperature of 105℃ (Pansu and Gautheyrou, 2006). Organic carbon was analyzed by Walkley Black method, it was then converted to organic matter by multiplying with Van Bemmelen factor of 1.742 (Jackson, 1973). Particle size was analyzed using particle analyzer Sympatrec T 100 laser diffraction granulometer, made in Germany.
Duplicate samples were taken for macrobenthic study to ensure precision. These samples were washed in the field itself through a sieve of mesh size 500 μm for macrofauna and those that are retained in the sieve were collected and preserved in 5% buffered formalin (Holme and Mc Intyre, 1971; McIntyre and Eleftheriou, 2005). Macrobenthic samples were sorted to different benthic groups by hand sorting. This was done in transparent plastic trays placed on a white back ground for easily distinguishing different benthic groups (McIntyre and Eleftheriou, 2005). Up to family or genus level identification was done for insects, molluscs and pisces (Yule and Sen, 2004; Munro, 2000). Identification was done up to species level for oligochaetes using taxonomic keys (Brinkhurst and Jamieson, 1971; Naidu, 2005). Identification was followed by a count of individuals per species (for oligochaetes) and groups (for other organisms) and the number was extrapolated to 1 m2.
1.3 Data analysis
The software programmes SPSS 16 (Statistical Programme for Social Sciences, version 16) and PRIMER 6 (Plymouth Routines in Multivariate Ecological Research, version 6) were used for statistical analyses. One way analysis of variance (ANOVA) was used to check the significant difference in different parameters between drought and flooded phases. Correlation analysis was done to check the relationship between environmental parameters and benthic abundance. Analysis of similarities (ANOSIM) was used to test statistically whether there is a significant difference between oligochaete compositions between drought and flooded phases. Univariate diversity indices such as species richness (Margalef’s index), species diversity (Shannon Wiener index) and species dominance (Simpson’s index) were computed for oligochaete species.
2 Results
2.1 Environmental parameters
The mean depth in drought phase was 0.36±0.14 m whereas it was 2.16±0.75 m in flooded phase. The results of ANOVA showed that there existed a significant difference in depth between phases (F1,53=138.70, p< 0.01). Sediment temperature was higher in the drought phase (27.66±3.35℃) than the flooded phase (26.88±1.00℃). The variation in sediment temperature was significant at between the phases (ANOVA F1,53=13.67, p< 0.01). Sediment pH was higher in the flooded phase (6.73±0.33) than the drought phase (6.45±0.44), the variation in sediment pH was significant (F1,53=6.93, p< 0.05). Organic matter showed no much difference between both the phases, an average value of 3.88±1.85% was recorded in drought phase and 3.83±1.10% in flooded phase. Despite of the different among phases, the moisture content remained similar in both the phases. The mean moisture content in drought and flooded phases were 29.73±8.36% and 29.95±5.43% respectively. The sediment was silty clay in drought phase and silty sandy in flooded phases.
2.2 Macrobenthic groups
The benthic fauna in Maranchery wetlands belonged to 4 phyla (Annelida, Arthropoda, Mollusca, Chordata) and 4 classes (Oligochaeta, Insecta, Gastropoda, Pisces). The faunal groups observed in drought phase were oligochaetes (24%), insects (75%) and molluscs (1%). In flooded phase oligochaetes (74.13%), insects (25.67%) and Pisces (0.20%) were the faunal groups (Figure 3). The class Insecta consisted of Diptera (true flies) represented by Chironomidae, Chaoboridae, Ceratopogonidae, Empedidae; Trichoptera (Caddisflies) represented by Gyrinidae, Limnephilidae; Hemiptera (True bugs) represented by Aphelecherinidae and Ephemeroptera (May flies) represented Baetidae. The insect fauna in wet phase consisted of Chironomidae (81.35%), Ceratopogonidae (11.86%), Chaoboridae (3.39%), Limnephilidae (1.69%) and Baetidae (1.69%) whereas that in dry phase consisted of Chironomidae (50.28%), Ceratopogonidae (22.34%), Gyrinidae (15.64%), Empedidae (3.39%) and Aphelecherinidae (2.79%). Molluscs were represented by Gastropods (Bithynia sp.) and Pisces present was Mystus sp.
Total macrobenthic abundance (355±122 ind./m2) and oligochaete abundance (263±473 ind./m2) was highest in flooded phase. Total macrobenthic abundance and oligochaete abundance in drought phase was 166±60 ind./m2 and 40±53 ind./m2 respectively. On the contrary insect abundance was highest in drought phase (124±168 ind./m2) than flooded phase (91±156 ind./m2) (Figure 4). ANOVA of total macrobenthic abundance showed no significant difference among the drought and flooded phases (F1,53=2.12, p>0.05) whereas oligochaete abundance showed a significant difference (F1, 53=5.11, p<0.05 ) among the phases. Abundance of insects also showed no significant difference (F1, 53=1.12, p>0.05) among the drought and flooded phases.
 Figure 4 Mean variation in numerical abundance (ind./m2) of macrobenthic faunal groups in drought and flooded phases |
The interactions between macrobenthic abundance and environmental parameters were analyzed using correlation analysis. The results showed no significant correlation between macrobenthic abundance and environmental parameters, the maximum correlation was observed between silt content and numerical abundance of macrobenthos (r2=0.13, p=0.35).
2.3 Composition and Community structure of Oligochaete species
Eighteen oligochaete species were identified from Maranchery wetland consisting of 14 species of the family Naididae, 3 of Tubificidae and 1 of Lumbriculidae (Table 1). Naididae (51%) and Tubificidae (49%) constituted the oligochaete families in the drought phase whereas Tubificidae (59%), Naidiae (40%) and Lumbriculidae (1%) constituted that in the flooded phase (Figure 5). Seven taxa; Pristinella jenkinae, Branchiodrilus semperi, Haemonais waldvogeli, Nais sp., Aulodrilus pluriseta, Aulodrilus pigueti, Aulodrilus sp. were present in both drought and flooded phases. Six species; Allonais gwaliiorensis, Dero dorsalis, Branchiodrilus hortensis, Pristinella acuminate, Nais andhrensis, Dero nivea were exclusively present in drought phase. Five species; Pristina breviseta, Pristinella minuta, Pristinella menoni, Aulophorus hymnae, Lumbriculus variegates were exclusively present in flooded phase. The most abundant species in both the phases were Aulodrilus pluriseta but their percentage of abundance was different, in the drought phase A. pluriseta constituted 31% of the oligochaete abundance whereas in flooded phase it constituted 50%. The composition of oligochaete species in the drought phase and flooded phase was compared using ANOSIM. The dissimilarity between the drought phase and flooded was not very strong (Global R=0.05, p>0.05).
In spite of the reduced numerical abundance in the drought phase, diversity analysis of oligochaetes revealed a slightly higher richness (d= 0.76) and diversity (H’= 2.96) values in the drought phase compared to that of flooded phases (d= 0.67, H’= 2.39). Dominance in the flooded phase (λ=0.29) was more in comparison to that of drought phase (λ=0.19) (Figure 6).
Discussion
There were many differences in the physical environment between the drought and flooded phases. Depth was the most variable parameter during this study. As the water body was shallow, a drop in few centimeters of depth implies the absence of water in the area. The low water level and the influence of the summer heat elevated the sediment temperature in drought phase. During flooded periods, water saturation results in more reduced soil and the consumption of free protons with reduction processes resulted in higher sediment pH in flooded phase (Stumm and Morgan, 1981; Langmuir, 1997). The decay of aquatic macrophytes and the influx of organic matter due to monsoon could be the reason for the high organic matter in the flooded phase. Whereas in the drought phase, though macrophytes and monsoon inputs were not there, the reduced water level would have concentrated the organic matter hence resulted in a higher organic matter level as observed by Real et al. (2000). The high organic matter content in both the phases could have resulted in similar moisture content in drought and flooded phases. High organic matter improves water holding capacity of sediments (Reddy and Patrick, 1998). The stagnant water in drought phase would have resulted in deposition of finer clay fractions in this phase. A quite condition, conducive for flocculation and settling of finer fraction is necessary for the deposition of clay (Nehru, 1990).
Flooded phase was a continuous water body providing vast habitable area for the aquatic organisms but the presence of water was restricted to few patches in the drought phase which restricted the presence of aquatic organisms in these remnant aqueous patches. When the continuous water body provided a freedom of movement or dispersal as in the flooded phase, the discrete patches in the drought phase prevented the same. The macrobenthic composition; the dominance of insects in drought phase and oligochaetes in the flooded phase clearly reflected the difference in the freedom of dispersal. The insects adopt a flight mode of dispersal or have active dispersal but oligochaetes are benthic crawlers or have passive dispersal (Bilton et.al., 2001). The habitat isolation and fragmentation have a less impact on insects due to their flight dispersal mode but it affected oligochaetes more that resulted in their reduced percentage composition in the drought phase.
The trend in numerical abundance in the drought and flooded phases clearly revealed the difference in habitable area between both the phases. In the drought phase the benthic samples were collected from the water patches, the only habitable area for the benthic organisms. All the living benthic organisms in that area would be available only in that water patch which guarantees the availability of benthos in the sample. But during flooded phases, the benthic organism could be present anywhere in the wetland substrata hence the chance of finding benthic organisms in our grab is less, so a strict comparison becomes difficult. The reduced habitable area in the drought phase resulted in a lower abundance in the drought phase and vice versa. When the area under inundation is increased, the habitable area increase and the number of organisms increase (Sommer and Horwitz, 2009). Most of the previous studies also documented an increase in benthic abundance with higher water levels (Cantrell, 1988; Gascon et al., 2007). In drought phase the reduced habitable area resulted in concentrating the benthic organisms to the available water patches. This limited habitable area lead to greater competition and other abundance-dependent effects resulted in the reduced numerical abundance in the drought phase. This finding is in agreement with that of Aspbury and Juliano (1998) who recorded that habitat desiccation result in decreasing the abundance of organisms as a result of greater competition and other abundance-dependent effects. Further in the drought phase, due to shallow nature of the water body, birds as well as other vertebrates and invertebrates and can access the water patches easily thus the threat of predation from them are more thus reducing the abundance. Sommer and Horwitz (2009) opined that drying wetlands concentrate aquatic prey for wading birds and mammals utilizing the wetland resulting in less numerical abundance.
The ANOSIM results revealed that the composition of oligochaete species remained similar in the drought and flooded phases. The peculiarities of oligochaetes could be the reason for this. oligochaetes are capable of surviving in dry conditions by specific mechanisms such as diapausing eggs; resistant cysts enclosing young, adults or fragments of individuals. Further they adopt a non-larval reproductive strategy, and therefore they do not rely on an open mouth state to recruit as would fauna relying on planktonic dispersal stages (MacKay et. al., 2010). So, the fragmented water patches that could restrict the planktonic larval distribution does not exert such an effect on oligochaete distribution. Both the properties of desiccation survival and non-larval reproductive strategy made oligochaetes survive in the drought phase. As the phases explored in this study were the same site in a different temporal scale the similarity in oligochaete composition is not surprising. Further in spite of difference in the physical structure between the phases, the similarity in oligochaete composition could be attributed to the less prominent niche specialization of oligochaetes (Verdonschot, 1989; Verdonschot, 1999).
In benthic communities disturbances have been related to changes in community parameters such as species richness, diversity and numerical abundance (Widdicomb and Austen, 2001) to changes in community structure (Warwick and Clarke, 1993). In the present study, the drought phase was under disturbance. Disturbances can be categorized by their temporal patterns as pulses, presses, and ramps. Ramps, which occur when the strength of a disturbance steadily increases over time (and often simultaneously in spatial scale), droughts are classified as ramp (Grigg, 1996). When the diversity was compared, the drought phase which was the disturbed phases showed a slightly higher diversity than the flooded or undisturbed phase. Dominance values were higher in the undisturbed phase than disturbed phase. Connel (1978) proposed the Intermediate Disturbance Hypothesis (IDH) to explain the high species diversity in rain forests and on coral reefs which was applied to other ecosystems later. He reasoned that there was a competitive hierarchy of species where, in the absence of disturbance, superior species would outcompete inferior ones, thus reducing species diversity. According to IDH theory, at intermediate levels of disturbance, diversity is maximized because both competitive K-selected and opportunistic r-selected species can coexist. The main difference between both K and r selected species is their growth and reproduction rate. In the case of oligochaetes, the life histories and reproductive strategies of naidids and tubificids vary considerably. Growth in naidid populations mainly depends on asexual reproduction (paratomy, fragmentation) within a short period of individual life spans, whereas sexual outbreeding plays only a minor role. Naidid populations are characterized by considerable fluctuations of abundance. Various species of naidids can disperse actively in the water column (Learner et al., 1978) and can thus more easily colonize different habitats. Due to these characteristics of naidids, they could be considered as r strategists. Aquatic and mesopsammic tubificids represent a 'conservative' type of sexual reproduction within well defined breeding periods and their population structure is fairly stable (Giere and Pfannkuche, 1982). Further as tubificids live mostly within the substrate, they are slow colonizers (Elissen et al., 2008; Levin et al., 1996). As these characters were ideal for K strategists, tubificids were considered as k strategists (Marchese and Ezcurra de Drago, 1992). Though the life strategy of naidids and tubificids arbitrarily fit their classification into r strategists and K strategists respectively, researchers opined that the classification of oligochaetes into r and K strategists is difficult. Further the distinction between r and K strategists is relative, since every species has a position in an r-K-continuum (Schaefer, 2003). According to Pianka (1970), an organism's position along r-K selection continuum depends on the particular environment at the particular instant in time. The classification of an organism as an "r-strategist" or a "K-strategist" is only relative to some other organism. Though oligochaetes are generally considered as r-strategists, within oligochaetes depending upon the colonization ability, reproductive strategy, population structure etc. Tubificidae are considered as K strategists and Naididae as r-strategists. In this study, in flooded phase, the family Tubificidae, which was considered as K strategists constituted 59% while in the drought phase their contribution was only 49%, where the Naididae (51%) which were r strategists also co occurred in large number. The slightly increased diversity in the disturbed phases compared to undisturbed phases reflects a glimpse of IDH theory, though not very prominent.
IDH theory also states that once K-selected and r-selected species can live in the same region, species richness can reach its maximum. The results of the present study also agree with this statement. The species richness in the disturbed phase (drought phase) was found to be more compared to that of the undisturbed phase (flooded phase). In undisturbed phase, the more uniform habitat pattern and the consistent environment might have resulted in the establishment of the characteristic species Aulodrilus pluriseta (A. pluriseta formed 50% of oligochaete species) resulting in high dominance and low diversity while in disturbed phases (A. pluriseta formed 31% of oligochaete species), the heterogeneity in the habitat and the changing environment would have resulted in modifying the species pattern ending up in high diversity. Gascon et al. (2007) compared the effects of hydrological disturbance on benthic communities in Emporda wetlands, Spain where he observed that in benthic communities, hydrological disturbance caused a decrease in dominance in the characteristic species. High diversity in dry phases compared to wet phase was observed in many studies. Contradictory results were also seen in some studies. Deeley and Paling (1999) stated that naturally variable systems are usually characterized by the dominance of pioneering species that are resilient to environmental fluctuations. Conversely, stable systems subject to minimal or infrequent disturbance generally support diverse communities with low species dominance.
The interactions between the environmental factors and benthic fauna based on correlation analysis showed that the benthic abundance was not significantly correlated to any of the environmental parameter. Though the relationship between benthos and the environmental parameters are clearly seen in majority of the studies, there are also studies where no relationship is observed between them (Prenda and Gallardo, 2007; Shobhana and Nair, 1983; Batzer, 2013). The absence of correlation in this study could be explained by the atypical nature of Maranchery kole wetland ecosystem and by the marked generalist character of the oligochaete species, the most abundant taxa in Maranchery. Niche discrimination in aquatic oligochaetes is less obvious than zoogeographic factors. Majority of these worms are adapted to live in sediments ranging from mud to sand. They survive in stony, sandy and muddy habitats, lowland rivers or lakes and ponds wherever soft substrates exist. Even the typical peculiarity of lacustrine and palustrine species which prevails in most of the aquatic organisms are also less evident in the case of oligochaetes (Thorp and Couch, 2001). Prenda and Gallardo (1992) documnented the ability of oligochaetes to colonize any kind of environment, from his observations in Mediterranean ecosystems where predictable wet and dry cycles exist. There was no significant relationship between oligochaetes and the physico chemical nature of the water in Veli lake (Shobhana and Nair, 1983). Aulodrilus pluriseta was the most abundant species present in Maranchery Kole wetlands, there are studies where the relationship between A. pluriseta and the environment remains ambiguous (Verdonschot, 1999; Nijboer et al., 2004). Chironomidae was the second numerically abundant taxa present. Like oligochaetes, chironomids also had a wide survival range ensuring its existence in a variety of environmental conditions. Random distribution of chironomid larvae was reported by McLachlan (1985) and Taylor (1961). This could be the reason for the absence of correlation of the benthic fauna and the environmental parameters. The pattern of ecological relationship evolved in this study reveals that some abiotic (e.g. habitat availability) or biotic variables (e.g. species interactions) that could cause the association with benthic fauna were not directly measured, or our variables were not good surrogates for them. Some factors which we have not measured but known to play a key role like the intensity of disturbance (Townsend and scarsbrook, 1997) hydrological stability, length of hydroperiod, (Williams, 2006), habitat duration (Williams, 1996), life history strategy (Wiggins et al., 1980, Williams, 1996) macrophyte density (Balcombe et al., 2005) area of the habitable patch (Anderson, 1998; Fleishman et al., 2002), proximity and size of the neighboring habitat (Russel, 2005), predation, wetland shape and size (Culler et al., 2013) etc. would have played a master role in the distribution of benthic fauna in spite of the physico chemical parameters. This suggests that the relationship with the measured environmental variables might be weaker or overridden by other unmeasured variables. Further every taxon does not respond to the same environmental variables, and especially not always in a linear way (Vlek et al., 2004).
Wetland destruction, global climate change, land use changes and water removal could lead to the shrinkage of water bodies to isolated water patches but its impact on the benthic community is less studied especially in the Indian context. As benthos form an important link in the food chain, the impact on them reflects the impact on its dependent organisms especially fishes, birds etc. Due to the vulnerability of these habitats to destruction, the scientific information from such contexts has to be gathered. The long-term solution of conserving wetlands lies in realizing the values and fragility of these systems and transmits that information effectively beyond scientific circles.
Authors contribution
S. Bijoy Nandan is the supervising guide, Rakhi Gopalan K.P made contributions in sampling and analysis.
Acknowledgements
The authors are thankful to the Head of the Department of Marine biology, Microbiology and Biochemistry, Cochin university of Science and Technology for providing necessary facilities. This study was a part of the research project funded by Kerala state Biodiversity Board, the authors are thankful to them. First author is thankful to University Grants Commission for the research fellowship.
References
Anderson M.J., 1998. Effects of patch size on colonisation in estuaries: revisiting the species-area relationship. Oecologia, 118:87-98
http://dx.doi.org/10.1007/s004420050706
APHA., 2005. American Public Health Association Standard Methods for the examination of analysis of water and waste-water 19th edition.
Aspbury A.S., and Juliano S.A., 1998. Negative effects of habitat drying and prior exploitation on the detritus resource in an ephemeral aquatic habitat. Oecologia, 115(1-2):137-148
http://dx.doi.org/10.1007/s004420050500
Balcombe C.K., Anderson J.T., Forney R.H., and Kordek W.S., 2005 Aquatic macroinvertebrate assemblages in mitigated and natural wetlands. Hydrobiologia, 541:175-188
http://dx.doi.org/10.1007/s10750-004-5706-1
Batzer D.P., 2013. The seemingly intractable ecological responses of invertebrates in North American wetlands: a review. Wetlands, 33:1-15
http://dx.doi.org/10.1007/s13157-012-0360-2
Bilton D.T., Freeland J.R., Okamura B., 2001. Dispersal of freshwater invertebrates. Annual Review of ecological systems, 32:159-81
http://dx.doi.org/10.1146/annurev.ecolsys.32.081501.114016
Brinkhurst R.O., and Jamieson B.G.M., 1971. Aquatic Oligochaeta of the World. Oliver and Boyd, Edinburgh.
Cantrell M.A., 1988. Effect of lake level fluctuations on the habitats of benthic invertebrates in a shallow tropical lake. Hydrobiologia, 158: 125-131
http://dx.doi.org/10.1007/BF00026271
Connell J.H., 1978. Diversity in Tropical Rain Forests and Coral Reefs Joseph Science, New Series, 199:1302-1310.
Culler L.E., Smith R.F., and Lamp W.O., 2013. Weak Relationships Between Environmental Factors and Invertebrate Communities in Constructed Wetlands. Wetlands.
Deeley DM, Paling EI (1999) Assessing the ecological health of estuaries in Australia. Institute for Environmental Science Murdoch University, LWRRDC Occasional Paper 17/99, Perth.
Eckman J., 1996. Closing the larval loop: linking larval ecology to the population dynamics of marine benthic invertebrates. Journal of Experimental Marine Biology and Ecology, 200:207-237
http://dx.doi.org/10.1016/S0022-0981(96)02644-5
Elissen H.J.H., Peeters E.T.H.M., Buys B.R., Klapwijk A., and Rulkens W., 2008. Population dynamics of free-swimming Annelida in four Dutch wastewater treatment plants in relation to process characteristics. Hydrobiologia, 605:131-142
http://dx.doi.org/10.1007/s10750-008-9329-9
European Environment Agency (EEA)., 2007. Climate Change and Water Adaptation Issues, EEA Technical Report No. 2/2007, Copenhagen, 110pp.
Ewers R.M., and Didham R.K., 2006. Confounding factors in the detection of species responses to habitat fragmentation. Biological Review, 81:117-142
http://dx.doi.org/10.1017/S1464793105006949
Fleishman E., Ray C., Sjogren-Gulve P., Boggs C.L., and Murphy D.D., 2002. Assessing the roles of patch quality, area and isolation in predicting metapopulation dynamics. Conservation Biology, 16:706-716
http://dx.doi.org/10.1046/j.1523-1739.2002.00539.x
Gascon S., Brucet S., Sala J., Boix D., and Quintana XD., 2007. Comparison of the effects of hydrological disturbance events on benthos and plankton salt marsh communities. Estuarine, Coastal and Shelf Science, 74:419-428
http://dx.doi.org/10.1016/j.ecss.2007.04.031
Giere O., Pfaankuche O., and 1982. Biology and ecology of marine oligochaeta, a review. Oceanography and Marine Biology Annual Review, 20:173-308.
Gopal B., and Zutshi D.P., 1998. Fifty years of hydrobiological research in India. Hydrobiologia, 384:267-290
http://dx.doi.org/10.1023/A:1003280426677
Grigg N.S., 1996.Water resources management. Principles regulations and cases. McGraw-Hill, New York.
Holme N.A., and Mc Intyre A.D., 1971. Methods for study of Marine Benthos, IBP Hand book No.6, Blackwell Scientific Publications.
Hughes J.B., Daily G.C., and Ehrlich P.R., 1997, Population diversity: its extent and extinction. Science, 278: 689-692
http://dx.doi.org/10.1126/science.278.5338.689
Humphries P., and Baldwin D.S., 2003. Drought and aquatic ecosystems: an introduction. Freshwater Biology, 48:1141-1146
http://dx.doi.org/10.1046/j.1365-2427.2003.01092.x
Hutchinson G.E., 1993. A Treatise on Limnology. Vol. 4. The Zoobenthos. New York: John Wiley & Sons.
IPCC (Intergovernmental Panel on Climate Change), 2007. Fourth Assessment Report. http://www.ipcc.ch/. Accessed 3 March, 2010.
Jackson M.L., 1973. Soil chemical analysis, Printers- Hall India Ltd, New Delhi.
Johnkutty I., and Venugopal V.K., 1993. Kole wetlands of Kerala, Kerala Agricultural University, Thrissur, 68PP.
Kumar P.S. and Khan A.B., 2013. The distribution and diversity of benthic macroinvertebrate fauna in Pondicherry mangroves, India Aquatic Biosystems, 9:15
http://dx.doi.org/10.1186/2046-9063-9-15
Kurup P.G., Varadachar V.V.R.G., 1975. Hydrography of Purakkad mud bank region Indian Journal of Marine Sciences, 4:18-20.
Learner M.A., Lochhead G., and Hughes BD., 1978. A review of the biology of the British Naididae (Oligochaeta) with emphasis on the lotic environment. Freshwater Biology, 8(4):357-375
http://dx.doi.org/10.1111/j.1365-2427.1978.tb01457.x
Levin L.A., Talley D., and Thayer G., 1996. Succession of macrobenthos in a created salt marsh. Marine Ecolgy Progress Series, 141:67-82.c
Mackay CF, Cyrus DP, Russell KL. 2010. Macrobenthic invertebrate responses to prolonged drought in South Africa’s largest estuarine lake complex. Estuarine, Coastal and Shelf Science, 86:553-567
http://dx.doi.org/10.1016/j.ecss.2009.11.011
Marchese M.R., and Ezcurra D.D., 1992. Benthos of the lotic environments in the middle Parana River system: transverse zonation. Hydrobiologia, 237:1-13
http://dx.doi.org/10.1007/BF00008422
Martins R.T., and Stephan N.N.C., and Alves R.G., 2008. Tubificidae (Annelida: Oligochaeta) as an indicator of water quality in an urban stream in southeast Brazil. Acta Limnologica Brasiliensia, 20:221-226.
McIntyre A.D., and Antasious Eleftheriou., 2005. 3rd edn. Methods for the study of Marine benthos. Blackwell Scientific Publications.
McLachlan A.J., 1985. What determines the species in a rain-pool? Oikos, 45(1):1-7
http://dx.doi.org/10.2307/3565215
Munro S.R., 2000. The marine and fresh water fishes of Ceylon. Published by Biotech Books, 349pp.
Naidu K.V., 2005. The Fauna of India and the Adjacent Countries-Aquatic Oligochaeta. Zoological Survey of India, Kolkata.
Nehru R.R., 1990. Ecology of macrobenthos in and around Mahendrapalli region of Coleroon estuary, south east coast of India, Ph.D. thesis, Annamalai University.
Nijboer R.C., Wetzel M.J., and Verdonschot P.F.M., 2004. Diversity and distribution of Tubificidae, Naididae and Lumbriculidae (Annelida: Oligochaeta) in the Netherlands: an evaluation of twenty years of monitoring data. Hydrobiologia, 520:127-141
http://dx.doi.org/10.1023/B:HYDR.0000027732.88238.61
Nkwoji JA, Yakub A, Ajani GE, Balogun KJ, Renner KO, Igbo JK, Ariyo AA , Bello BO. 2010. Seasonal variations in the water chemistry and benthic macroinvertebrates of a south western Lagoon, Lagos, Nigeria. Journal of American Science, 6(3):85-92.
Pandit A.K., Qadri M.Y., and Jan U., 1991. Benthic communities of inland waters of India. In Current Trends in Limnology. Vol. 1. N. K. Shastree (ed.), Narendra Publishing House, New Delhi, 237-250.
Pansu M., and Gautheyrou J., 2006. Handbook of soil analysis - Mineralogical, organic and inorganic methods, Springer, Berlin, Heidelberg, New-York, 993 pp.
Pianka E.R., 1970. On r- and K-selection. American Naturalist, 104:592-597
http://dx.doi.org/10.1086/282697
Pimm S.L., Russell G.J., Gittleman J.L., and Brooks T.M., 1995. The future of biodiversity. Science, 26: 347-350
http://dx.doi.org/10.1126/science.269.5222.347
Prenda J., and Gallardo A., 1992. The influence of environmental factors and microhabitat availability on the distribution of an aquatic oligochaete assemblage in a Mediterranean river basin. International Review of Hydrobiology, 77:421-434
http://dx.doi.org/10.1002/iroh.19920770306
Real M., Rieradevall M., and Prat N., 2000. Chironomus species (Diptera: Chironomidae) in the profundal benthos of Spanish reservoirs and lakes: Factors effecting distribution patterns. Freshwater Biology, 43:1-18
http://dx.doi.org/10.1046/j.1365-2427.2000.00508.x
Reddy K.R., and Patrick W.H., 1998. Biogeochemistry of wetlands. University of Florida, Gainesville, Florida, USA.
Russell B.D., Gillanders B.M., and Connell S.D., 2005. Proximity and size of neighboring habitat affects invertebrate diversity. Marine Ecology Progress Series, 296:31-38
http://dx.doi.org/10.3354/meps296031
Schaefer M., 2003. Wörterbuch der Okologie. 4. Auflage. Heidelberg/Berlin: Spektrum Akademischer Verlag, 452 pp.
Sobhana S., and Nair N.B., 1983. The biocoenosis of Salvinia mo- lesta Mitchell in theVeli Lake, southwest coast of India. Areport on the associated oligochaetes. Proceedings of Indian National Science Academy, B49:101-107.
Sommer B., and Horwitz P., 2009. Macroinvertebrate cycles of decline and recovery in Swan Coastal Plain (Western Australia) wetlands affected by drought-induced acidification Hydrobiologia, 624:191-203
http://dx.doi.org/10.1007/s10750-008-9692-6
Suriani-Affonso A.L., França R.S., Marchese M., and Rocha O., 2011. Environmental factors and benthic Oligochaeta (Annelida, Clitellata) assemblages in a stretch of the Upper Sao Francisco River (Minas Gerais State, Brazil). Brazilian Journal of Biology, 71(2):437-446.
Taylor L.R., 1961 Aggregation, variance and the mean. Nature (London) 189:732-735
http://dx.doi.org/10.1038/189732a0
Thienemann A., 1924. Hydrobiologische Untersuchungen an Quellen - Archives of Hydrobiologia, 14:151-190.
Thorp J.H., and Covich A.P., 2001. Ecology and Classification of North American Freshwater Invertebrates. Academic Press, New York, 1056 pp.
Townsend C.R., and Scarsbrook M.R., 1997. The intermediate disturbance hypothesis, refugia, and biodiversity in streams. Limnology. Oceanography, 42:938-949
http://dx.doi.org/10.4319/lo.1997.42.5.0938
Verdonschot P.F.M., 1999. Micro-distribution of oligochaetes in a soft-bottomed lowland stream (Elsbeek; The Netherlands) Hydrobiologia, 406:33-47
http://dx.doi.org/10.1023/A:1003795311154
http://dx.doi.org/10.1023/A:1003796403364
Verdonschot P.F.M. 1989. The role of oligochaetes in the management of waters. Hydrobiologia, 180: 213-227. doi: 10.1007/BF00027554.
Vijayan V.S., Prasad S.N., Vijayan L., and Muraleedharan S.C., 2004. Inland wetlands of India- Conservation priorities. Salim Ali Institute for ornithology and natural history.
Vlek H.E., Verdonschot P.F.M., and Nijboer R.C., 2004. Towards a multimetric index for the assessment of Dutch streams using benthic macroinvertebrates. Hydrobiologia, 516:173-189
http://dx.doi.org/10.1023/B:HYDR.0000025265.36836.e1
Warwick R.M., and Clarke K.R., 1993. Increased variability as a symptom of stress in marine communities. Journal of Experimental Marine Biology and Ecology, 172:215-226
http://dx.doi.org/10.1016/0022-0981(93)90098-9
Widdicombe S., and Austen M.C., 2001. The interaction between physical disturbance and organic enrichment: An important element in structuring benthic communities. Limnology and Oceanography,46:1720-1733
http://dx.doi.org/10.4319/lo.2001.46.7.1720
Wiggins G.B., Mackay R.J., and Smith I.M., 1980. Evolutionary and ecological strategies of animals in annual temporary pools. Archiv für Hydrobiologie, Supplement, 58:97-206.
Williams D.D., 1996. Environmental constraints in temporary freshwaters and their consequences for the insect fauna. Journal of the North American Benthological Society,16:634-650
http://dx.doi.org/10.2307/1467813
Yule C.M., and Sen Y.H., 2004. Freshwater invertebrates of the Malaysian region / editors Kuala Lumpur, Malaysia : Akademi Sains Malaysia.
. PDF(527KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Vineetha S.
. Bijoy Nandan S.
. Rakhi Gopalan K.P.
Related articles
. Oligochaetes
. Kole wetlands
. Benthos
. Diversity
. Flooded phase
. Drought phase
Tools
. Email to a friend
. Post a comment


.jpg)




